Far from a niche industry, the orphan drug market is now ripe with opportunities for pharmaceutical companies. According to the National Institutes of Health, around 10% of the US population lives with a rare disease – contributing to a growing global market with an estimated worth of $340.84 billion by 2027. Medical affairs sits at the intersection of R&D and commercial operations, and as such, is best placed to capitalize on the opportunities the orphan drug market has to offer. In this article, we’ll explore two key areas where insights management technology can support orphan drug development and medical affairs.
Clinical trial design
Clinical trial design can be a complicated beast at the best of times, but it’s an order of magnitude more complex when rare diseases are involved. Challenges include identifying patient populations – given each rare disease affects a maximum 200,000 people in the US – and ensuring participants are sufficiently diverse and representative of the general population. Site identification can be equally challenging, with patient populations likely to be spread sparsely across broad geographic areas – potentially, even across national borders. Providing sufficient site access for these individuals, some of whom may have mobility issues or financial concerns that could curb travel, can be an insurmountable challenge.
“By necessity, many rare disease clinical trials are multicentre, often multinational, for sufficient patient recruitment, even in phase I and II trials… Language differences and working across varied time zones can add complications.” – British Journal of Dermatology
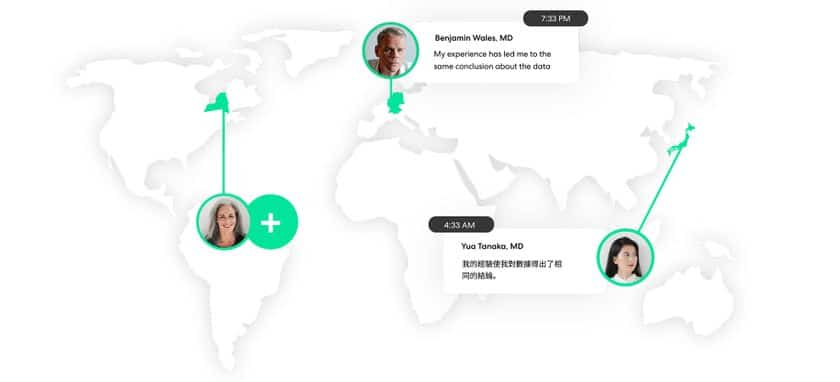
Here, insights management technology is poised to alleviate some of the most formidable barriers in a plan for orphan drug development. Asynchronous engagement applications allow trial teams and patients to connect across geographies, time zones, and even languages. It’s a tool that effectively enables some aspects of decentralized clinical trials – minimizing or removing the need for in-person interaction and logistically challenging clinical visits. Instead, patients can connect with clinicians remotely, in their language, and at a time and place that works for them.
Social listening can also be an effective tool in clinical trial design. Social listening taps into the online chatter of patients, HCPs, and other experts – providing insight into what they think and feel. This window into the patient experience can help medical affairs teams design more patient-centric clinical trials and, therefore, be more likely to meet enrollment targets and minimize dropouts.
Although medical affairs organizations may already employ these tools separately or with some degree of connectedness, the reality is that most life science companies still use manual analysis across most of their insight-generation activities. Teams with a more mature insights management approach use group engagement applications and social listening in a single platform, where they can relate to the same key insight questions and higher-level goals.
Medical education
A key role of medical affairs is to liaise with external HCPs and experts and return insights to internal colleagues. The department is a conduit for medical education both within an organization and externally via the production and distribution of medical education materials and through highly trained MSL teams.
Medical education is of particularly vital importance in orphan drug development. Due to the nature of rare disease clinical trials, most pharma companies will have little or no experience with the therapeutic area they’re launching into when developing a new orphan drug. Teams are unlikely to be plugged into these rare disease communities and won’t be able to build on a catalog of knowledge and experience as they might in other, more common therapeutic areas. Medical affairs plays a crucial role in bringing organizations up to speed.
“Usually, when a large company introduces such a treatment, it is entering the relevant therapeutic area for the first time. It is therefore likely to lack both expertise in the disease and in-depth understanding of the health ecosystem and of patients’ experience of the disease.” – McKinsey
An insights management platform dramatically enhances medical education processes for med affairs teams. Tools like artificial intelligence, natural language processing, and sentiment analysis optimize many resource-intensive processes associated with field force insight gathering. Natural language processing can rapidly identify trends and surface key insights for immediate assessment. Sentiment analysis, meanwhile, can parse these trends for sentiment – effectively revealing what an HCP thinks or feels about a particular topic. This way, medical affairs teams can rapidly gather critical insights from MSL interactions for effective dissemination across the organization.
Asynchronous engagement also serves as a compliant venue for collaboration and discussion. Vital patient insights can be gathered through advisory boards composed of patients and patient advocates, while features such as document co-authoring allow education materials to be shared and worked on collaboratively – all in a secure, fully-compliant environment.
The orphan drug market is complex and allows little margin for error, but there are opportunities in this growing market sector. Medical affairs is uniquely positioned to capitalize on these opportunities, and insights management technology is ready to help drive success.