Our previous article on rare diseases and orphan drug development discussed how the orphan drug market represents a growing opportunity for pharmaceutical companies. However, specific rare diseases are still niche disciplines in which relevant expertise can be hard to acquire. In this article, we discuss the orphan drug ‘people problem’, and explore how medical affairs teams can find and engage orphan drug experts and investigators.
What is the rare disease ‘people problem’?
A successful drug launch strategy depends on thoroughly understanding the therapeutic area and disease community a proposed new treatment will launch into. In the orphan drug market, such understanding is rare indeed. “Usually, when a large company introduces such a treatment, it is entering the relevant therapeutic area for the first time,” reports McKinsey. “It is therefore likely to lack both expertise in the disease and in-depth understanding of the health ecosystem and of patients’ experience of the disease.”
It is estimated that 95% of rare diseases have no treatment options. There aren’t the same communities of investigators, HCPs, and experts as we’re used to from more common therapeutic areas. This lack of in-depth understanding affects the entire drug development process, from top to bottom.
What are the challenges of finding rare disease experts?
- Limited investigator numbers. By definition, rare diseases affect extremely small patient populations. As such, there are limited numbers of investigators with experience in treating each disease, and their experience may be equally limited.
- Limited investigator knowledge. In many cases, rare disease patients and their carers or advocates have a greater knowledge of their conditions than the medical community. It’s rare to find investigators with complete awareness of a particular rare disease treatment landscape, the patient experience, and the available treatment options – if any.
- Geographical dispersion. Finding sufficient patient numbers to participate in a rare disease trial can be enormously challenging and require decentralized trials spread across large geographical areas – even national borders. The same is true of trial investigators.
- Trial complexity. Rare disease trials can be enormously complex – involving multiple, widespread trial sites, requiring highly specialized expertise, and necessitating numerous concessions for patients with highly-specific needs. This complexity can act as a disincentive for investigators to participate.
- Funding restrictions. Similarly, rare disease research funding can be limited or difficult to obtain, making attracting and retaining investigators even more challenging.
In a nutshell, rare disease trial investigators are small in number, hard to find, attract, and retain – even if they possess the expertise required to make the orphan drug development process a success.
How technology can help
Orphan drug development is challenging, and no amount of technological intervention can conjure rare disease experts where none can be found. However, an insights management platform can help pharma companies reveal the hidden experts and influencers within a rare disease community and engage them throughout the drug development process.
Finding the right people
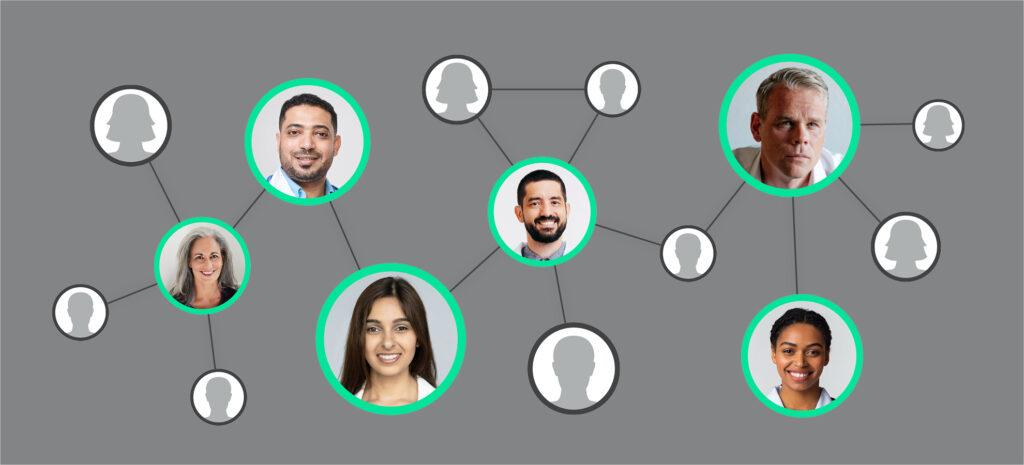
The true trust brokers within a disease community are no longer necessarily the same people who publish the most literature or speak at the most medical congresses. Now, pharma teams can look for a disease community’s ‘invisible college’ – hidden networks of HCPs, experts, patient advocates, and patients. These influential individuals aren’t necessarily – or even very often – the same names as appear at the top of pharma teams’ engagement lists.
Network analytics can help life science teams uncover this invisible college. Network analytics investigates numerous data sources – such as medical claims, social media, and scientific data – to uncover the most influential individuals within a disease community and reveal how they’re connected.
“Those companies that have successfully launched rare disease drugs have discovered the vital role that the rare disease community – patients and their families, advocacy groups, and a small number of therapeutic area experts (TAEs) – plays in generating these insights.” – McKinsey
Social listening in pharma is another potentially valuable tool in orphan drug development. Social listening monitors online chatter around a particular condition, treatment, congress, or pharma company. It surveys blogs, social networks, forums and other online communities to provide valuable disease community insights. It’s a crucial window into the patient experience that pharma teams can use to create more effective, patient-centric trial experiences.
Engaging the right people
In a therapeutic area where every expert counts, pharma teams should make it easier for investigators and KOLs to engage. Asynchronous virtual engagement can remove many barriers to some aspects of trial participation for patients and experts. It effectively enables decentralized clinical trials, allowing participants to engage at a time and place that works for them – regardless of schedule conflicts or geographical barriers. In-platform translation functionality means participants can engage in their languages, allowing pharma teams to engage experts worldwide and collaborate across the language barrier effortlessly. Effectively, asynchronous virtual engagement technology rolls out the red carpet for a rare disease community’s sought-after KEEs and KOLs.
Overcoming the orphan drug ‘people problem’ is just one piece in the drug development jigsaw puzzle. Read the first post in this series for more information on how insights management technology can support orphan drug development. Ready for more? Read the third and final post in the series.