Life science organizations need input from external clinical advisors such as healthcare providers like physicians, pharmacists, and nurses – even patients and caregivers are emerging as vital sources of medical information who can have a significant impact on strategic decisions. Management of these external relationships falls to medical affairs teams.
What is medical affairs in the pharmaceutical industry? The medical affairs role concerns itself with post-approval product activities. Through external advisors, medical affairs teams provide scientific and clinical support commercial teams need to foster access to, and adoption of, new diagnostic tools, therapies, devices and other technologies that improve patient outcomes.
What are the skills needed for a medical affairs team member?
According to McKinsey’s Vision for Medical Affairs report, a medical affairs professional or team is considered the natural owner of scientific data across the product lifecycle. Medical affairs skills for success within a life science organization include
- Medical credentials or equivalent education
- Scientific know-how
- Credibility among internal and external stakeholders
In the medical community, the profession has largely left behind its former status as a support function and has forged a new role as a primary strategic pillar of life science organizations alongside clinical research and clinical development, commercial, and market access functions. Innovation in medical affairs insight gathering can impact every phase of the product development lifecycle, so it’s critical that feedback from experts is measured against established medical affairs KPIs and shared with leadership and across other functional teams.
What are the responsibilities of a medical affairs team?
Common deliverables for a medical affairs personnel team include:
- Evidence generation
- Articulation of a medical product or technology’s clinical and economic value
- Decision-making support for clinicians and patients
- Provision of strategic medical direction to the organization, including senior leadership
These teams are also responsible for adhering to medical affairs compliance requirements.
What do medical affairs teams need to do their job effectively?
Life science organizations make substantial investments in establishing and strengthening relationships with their external advisors, and in medical affairs teams that support commercial operations. The more effectively medical affairs can extract actionable insights and data from KOLs, MSLs, and other experts, the more likely they are to gain market share, improve patient access, or pivot if needed.
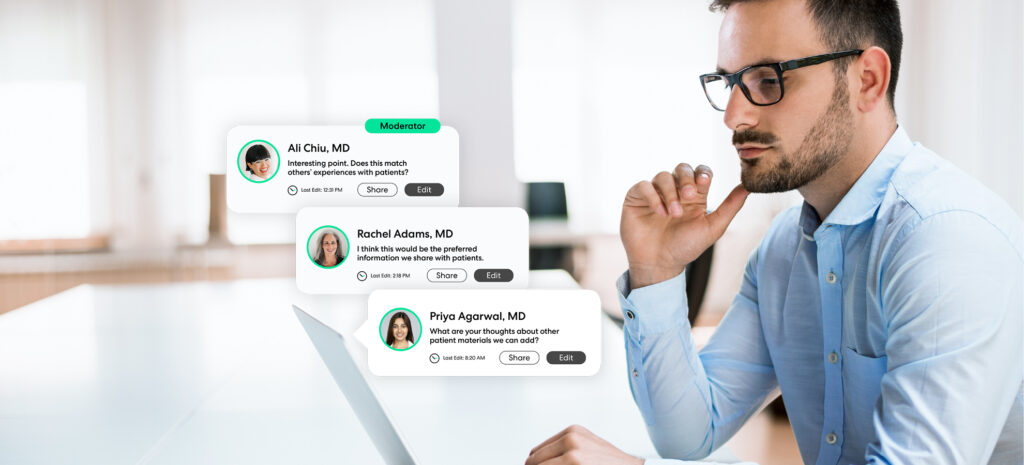
At a minimum, medical affairs teams need to engage external stakeholders in a way that works for them, and optimally in a way that cultivates enthusiastic, eager contributors to the organization’s projects. As more life science teams embrace digital transformation, engagement methodologies have expanded to include virtual interactions and connections with new audience types.
A paradigm shift in KOL engagement for medical affairs
The events of early 2020 created a shift in how a medical affairs professional engages with external stakeholders. The days of key experts attending numerous in-person, one or two-day advisory board meetings are likely over now that virtual tools prioritize convenience over face-to-face interaction for every meeting.
Now that some in-person interaction will be possible again, hybrid engagements – a combination of asynchronous discussions with real-time virtual meetings such as webcasts – have become the gold standard for medical affairs KOL engagement. Placing more emphasis on convenient and accessible digital insight-gathering methods increases diversity, promotes cross-functional connectivity, and invites more comprehensive feedback.
If it’s true that KOLs will be less interested in travel, pharmaceutical and medical device teams should consider how to offer a more tailored experience that will help them establish and strengthen those important KOL connections. Within3 clients find that they can prioritize KOLs’ schedules while obtaining deeper, more relevant insights by leveraging asynchronous and hybrid virtual engagement, such as a virtual advisory board. While virtual will never entirely replace traditional, in-person gatherings, a virtual-first strategy offers more options and yields better results than exclusively face-to-face engagements or video calls alone.
As circumstances at work and home return to a semblance of pre-pandemic normalcy, life science teams will retain whatever helps them work more efficiently. By focusing on the audience, augmenting face-to-face engagements with virtual elements, and choosing a platform that speeds the pace of work, medical affairs teams should prioritize a virtual-first strategy.
To learn how medical affairs teams use hybrid virtual engagement to increase insight-gathering capabilities and obtain more actionable information, read a recent customer success story.