APPLICATION
Better engagement for better clinical trial performance.
Within3’s insights management platform delivers seamless collaboration and communication for clinical trial design and operations teams. Sponsors engage sites, patients, and payers to improve study design, training, and trial execution.
APPLICATION TRAINING AND KNOWLEDGE ASSESSMENT
On-demand training
Allow sites to access training resources on their own schedule and ensure that training is complete and successful, without concerns about version control or outdated documentation that can throw critical timelines off track.

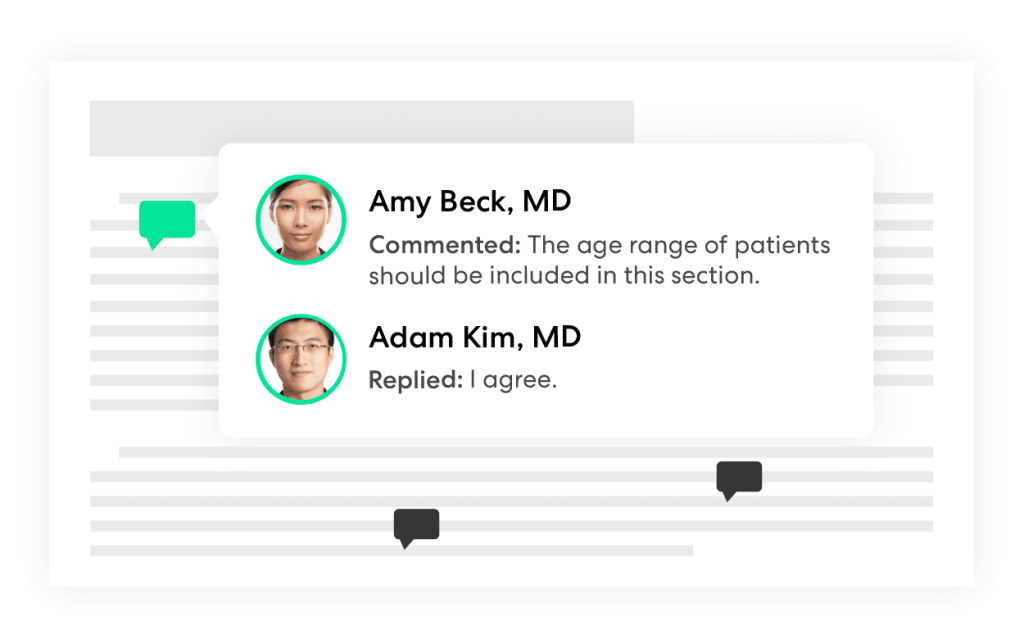
DOCUMENT ANNOTATION AND CO-AUTHORING
Design trial protocols and materials
Co-create or gain critical feedback on all documents and materials related to a trial. Gain insights from all relevant stakeholders, including patients, to improve trial outcomes.
VERSATILE PLATFORM
Engage all crucial stakeholders
Engage patients, payers, investigators, and site staff to ensure that clinical trial design and operations are optimized for traditional and decentralized clinical trials.
CLIENT SUCCESS
Read how a clinical development team improved clinical trial design with investigator feedback.
Business Results
Improved trial execution
Gain real-time feedback from sites, patients, and payers before or during a study to improve trial performance.
Fewer logistical headaches
Easily host global discussions with instant translation, mobile compatibility, and 24/7 environment for clinical trial design and operations teams.
Smarter decisions
Engaging HCPs, patients, and payers for feedback means better decisions throughout this clinical trial—and the next one.





