Pharma teams know they need to be more nimble – changing strategy based on new data or emerging trends is critical to success. According to medical affairs leaders responding to our insights management survey, pharmaceutical companies could face product launch risk and other problems without this ability.
What’s interfering with agility?
When we asked medical affairs teams to tell us what they needed to be more agile, they chose a variety of answers. Among the top responses:
- 66% of medical affairs leaders said they need more aligned internal communications and updates
- 59% said they need more information and better insights from the data they collect
- 48% would consider themselves more agile with the help of better technology
- 46% see a need to demonstrate faster reaction times
Whatever the obstacle, the results are clear: medical affairs teams are putting their strategies at risk when they’re not agile and aligned. And at the heart of this misalignment is a lack of insights and a need for technology supporting medical affairs.
The price of falling behind
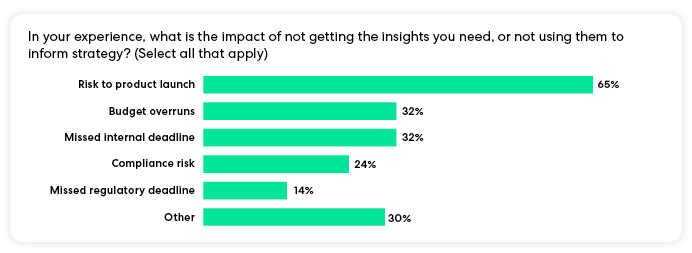
If one of medical affairs’ most pressing problems is limited agility due to a lack of insights, we wanted to know: what do medical affairs leaders consider the biggest consequence of not having the insights and agility they need?
Risk to product launch was the most frequently cited impact of not getting insights, with 65% of respondents. Other top concerns included budget overruns, missed internal deadlines, compliance risks, and missed regulatory deadlines.
But medical affairs leaders also described the impact in their own words:
“Wasted internal resources spent on insight generation not used for strategic decisions.”
“Delayed evidence generation, delayed reactions to evolving needs.”
“Risk to clinical trial strategy.”
But perhaps the most frequent response called out missed opportunities, including the opportunity to improve value to customers and to reach specific market segments. In short, medical affairs teams and their organizations may leave much on the table without the right insights – including the chance to serve customers better or identify new markets for their treatments.
Solving the agility problem
The stakes for pharmaceutical organizations are high. Some unforeseen factors can derail a pharma product launch – regulatory issues, macroeconomic factors, and competitor activity, to name a few – but ultimately, a lack of insights is a problem that can be solved.
Our survey indicates that medical affairs teams using a single, central way of managing insights, such as an insights management platform, overwhelmingly believe that it helps them work more effectively. Gaining the ability to separate signal from noise is paramount in an environment of ever-accumulating data from an increasing number of channels.
To see more results from the insights management survey, read the survey data report or get our insightful white paper featuring experts from Merck, Pfizer, Astellas, Spark Therapeutics, Within3, and MAPS. Download the white paper.